Allegra


A brief guide to Advair Diskus Side Effects
People who have asthma may be prescribed Advair Diskus if their current prescription is not efficient in alleviating their symptoms. The side effects of Advair Diskus; however, can be serious. As a result, patients must monitor their intake to ensure that the long or short term effects of Advair do not arise.
This drug is not intended to be taken indefinitely; when taken over an extended period of time, the risk of Advair Diskus side effects increase greatly. One of the most serious potential side effects of Advair Diskus–that increases with time is–an asthma-related death. To avoid serious Advair side effects, your doctor will decrease and/or discontinue your intake following the dissipation of your symptoms.
Before you are prescribed the drug, a doctor must explain all potential Advair Diskus side effects. Some effects are not unique to the medication. More common side effects of Advair Diskus are less serious, including headaches and throat irritation. Although the bulk of adverse reactions should be reported, minor or common effects do not necessitate medical intervention.
More serious immediate Advair Diskus side effects include allergic reactions. If you develop a rash or hives, you should immediately stop taking the drug. Advair long term effects include the risk of developing pneumonia or other sicknesses. It is vital that you report any such Advair Diskus side effects immediately. Though meant to help asthma patients, the effects may not begin to occur for a week. If you notice side effects of Advair Diskus your asthma is worsening.
Children who take this drug risk Advair long term effects related to growth; the drug can stunt children's growth. To monitor such Advair long term effects, children's growth should be regularly monitored by a doctor. Similarly, the potential side effects of Advair Diskus for adults include problems with bone mass density. Left untreated, these Advair long term effects could result in osteoporosis.
All treatment will begin with the smallest possible dose of 100 micrograms and be increased until it proves effective. To avoid Advair long term effects, you should discontinue use as directed by a physician.
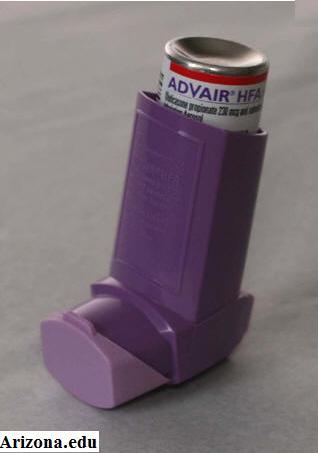
A brief guide to Advair 250 inhalers
The medication Advair is prescribed help alleviate severe asthma symptoms. Additionally, it can be issued for long-term use among patients who have emphysema, bronchitis or other chronic pulmonary disorders. An Advair 250 inhaler is issued to administer the medication.
It is unsafe to take apart an Advair 250 inhaler or otherwise tamper with it. To ensure that you are safely storing Advair 250 inhalers, make sure the device is placed in a cool and dry area with a temperature between 68 and 77 degrees Fahrenheit. Make sure that the Advair 250 inhaler cannot be accessed by children, for the medication should not be ingested nor used by children below the age of four.
Unlike the shape of a standard emergency inhaler, an Advair 250 inhaler is disk-like. To ensure safe use, Advair 250 inhalers should be stored in a safe place where they cannot be tampered with. The medication is administered in a dry powder form. When you ingest the medication through an Advair 250 inhaler, you may not feel the powder enter your mouth. Regardless of this feeling, it is imperative that you do not ingest another dose.
Following ingestion, you should hold your breath for 10 seconds, and then rinse with water for an additional 10 seconds. Do not swallow the liquid after making use of the Advair 250 inhaler. Doses should be taken twice daily, with a 12 hour separation.
Advair 250 inhalers come with a counter to indicate how many doses are left. When running low, the Advair 250 inhaler will display the remaining doses in red. This indicates that you must schedule an appointment with your physician to refill your prescription. At this time, you should report any discomfort or adverse effects you experience.
Advair 250 inhalers are not intended for the emergency relief of sudden breathing problems. You should use your emergency inhaler in case you experience any such attacks. If you find yourself short of breath after using an Advair 250 inhaler and do not obtain relief from an emergency inhaler, obtain medical treatment immediately.
Report any adverse effects no matter how severe. In addition to immediate breathing problems, skin problems such as rashes or hives may indicate that you are having an allergic reaction to the medication from Advair 250 inhalers. Be alert to eye blurriness, since the medication can lead to glaucoma, cataracts and similar problems.
A physician who fails to take appropriate precaution when you report such problems may be guilty of malpractice. If so, you may have grounds for litigation related to serious side effects which you experience. Consult with a lawyer who is experienced in malpractice lawsuits who can evaluate your medical records and determine whether a doctor was negligent in supervising your treatment.