Actonel Dosages
A Brief Guide to Actonel Dosages
The prescription medication Actonel is prescribed to patients who wish to maintain healthy bone mass and structure. The Actonel dosage prescribed by a doctor should follow the guidelines issued by its manufacturer. In order to prescribe the correct Actonel dose, a physician must consider your medical condition, state of health and any other medications (prescription and non-prescription) you are taking.
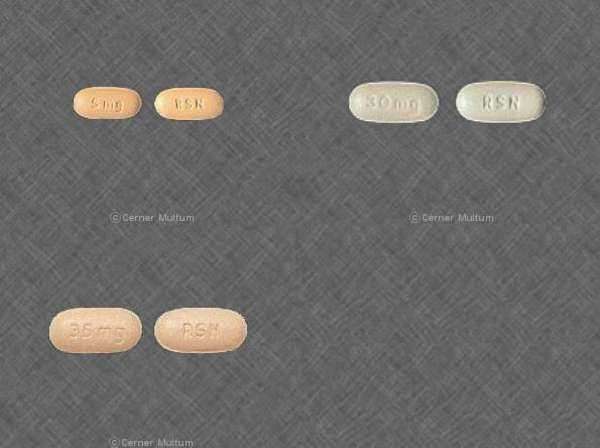
There are five different Actonel dosages, all of which are administered in tablet form. In nearly all treatment plans, you may be issued an Actonel dosage in the form of a 5 milligram (mg) tablet to be taken daily, a 35 mg tablet to be taken weekly, a 75mg mg tablet which should be taken for two consecutive days once a month, or a 150 mg tablet taken once a month.
The specific Actonel dose you will be described is primarily issued in relation to your specific wants or goals. For the prevention or treatment of osteoporosis in postmenopausal women, any of these Actonel dosages may be considered by a doctor. In the case of men with osteoporosis who wish to increase their bone mass, the recommended Actonel dosage is a weekly 35mg tablet. However, men who are battling osteoporosis related to steroid use should be issued an Actonel dose in the daily 5 mg form.
Along with osteoporosis-related treatment, Actonel dosages are considered for men and women battling Paget's disease. This condition can lead to deformed or enlarged bones. In such cases, the recommended Actonel dosage takes the form of a 30 mg pill. This Actonel dose should be taken daily for two months. Once this treatment has concluded, such Actonel dosages may be repeated if no improvement is observed. Studies have been inconclusive as to what kind of Actonel dosage should be administered if this second round of treatment is not effective.
Regardless of the Actonel dose you are prescribed, it should be taken 30 minutes before eating your first meal of the day. All Actonel dosages should be consumed with nothing more than water. Calcium supplements may be necessary during the onset of your treatment plan.
Regardless of your specific Actonel dose, it is important to be alert to any indication of adverse effects. Any Actonel dosages can result in potentially serious conditions. The most serious potential side effect with Actonel dosages is osteonecrosis of the jaw. It is crucial that you report any pain around your mouth to a physician immediately. If left untreated, an Actonel dose which causes these kinds of side effects could lead to debilitating pain as well as the loss of your jaw.
A physician should clearly explain all potential side effects of Actonel dosages before you begin a course of treatment. In the event that you are seriously harmed by your Actonel dosage, you may have grounds for a lawsuit to recover medical expenses or damages regarding pain and suffering. Many such lawsuits have been brought forth by patients who have taken every form of Actonel dose.
Actonel Class Action Lawsuit

How to join an Actonel class action lawsuit:
The prescription medication Actonel has been associated with several severe side effects. Most notably, patients who have suffered from osteonecrosis of the jaw may be able to join an Actonel class action suit. Actonel class action lawsuits for this particular side effect include hundreds of patients who suffer from “jaw bone death” as a result of Actonel use. So, because you are joining those who share similar stories, you do not have to independently file a claim against Actonel.
Generally, the defendant in an Actonel class action lawsuit is the manufacturer of the drug, Warner Chilcott Laboratories. Sufferers damaged by the drug's side effects may not always be eligible to join an Actonel class action suit; the individual must endure the same symptoms or be diagnosed with the same medical condition as the other plaintiffs in the Actonel class action suit.
Some of the Actonel’s side effects are common to other medications and will probably not help establish an Actonel class action lawsuit. For example, difficulty sleeping or diarrhea will not provide sufficient grounds to form an Actonel class action suit. Significant, long-lasting medical damage must be the foundation of Actonel class action suits. Since osteonecrosis of the jaw begins with oral pain and can end in the removal of your jaw, it qualifies as grounds for an Actonel class action lawsuit.
If you have been hurt Actonel use, you must immediately consult with your physician to either reverse the reaction or document your condition. In the latter situation, you may be able to join an Actonel class action suit without committing significant resources. Even though these kinds of cases consolidate several plaintiff complaints, only one person is required to represent the group. This person serves as the primary liaison to the defense team.
If you learn that an Actonel class action suit is already being undertaken, you must contact the representing law firm. You should then schedule a meeting to discuss adding your name to the list of plaintiffs in an Actonel class action lawsuit. When meeting with the facilitator, you must present all medical records to document the adverse reacts. This information will serve as the foundation for your Actonel class action suit. The more evidence delivered to the lawyer the more effective the professional will be in proving negligence or fault on the part of the drug maker.
Depending on the number of plaintiffs involved, resolving an Actonel class action lawsuit can vary in time; some Actonel class actions suits can take years while others will be wrapped up in a couple of short months. Commonly, an Actonel class action suit may be handled by more than one law firm. This is necessary to handle the large volume of documentation involved in an Actonel class action.
You should be kept informed if you request about all progress made in an Actonel class action lawsuit. However, once you have provided your records, you will probably not need to appear in court or directly involved yourself in an Actonel class action suit. You should be notified if and when an Actonel class action results in a financial settlement to be divided among plaintiffs.
Zyprexa

HeartWare Ventricular Assist System Approved
On November 20, the Food and Drug Administration announced the approval of the HeartWare Assist System that helps support heart function and blood flow for patients who are at the end-stage of heart failure and currently await a transplant.
The newly approved Assist System is a left ventricular assist device (LVAD)—the most common ventricular assist devices—but the newly approved LVAD is unlike other assist devices in the past. Former LVADs come with components that need to be planted in the patient’s abdomen, but the new HeartWare System is small enough to fit in the chest cavity near the heart. The new system provides specific advantages to smaller patients who cannot receive the abdomen implantation.
The HeartWare LVAD was approved after a clinical trial called the ADVANCE trial. 137 patients with advanced heart failure were analyzed and compared to other patients in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). The advantages of the HeartWare LVAD showed more benefits than complications.
There are serious possible complications associated with the new LVAD though. Serious infections and strokes can occur when using the new HeartWave LVAD.
The approval of the new LVAD is the first time the FDA has approved an LVAD with the INTERMACS system as a control. The system is managed by the University of Alabama and represents a joint effort of the FDA, National Heart, Lung and Blood Institute (NHLBI), the Centers for Medicare and Medicaid Services (CMS), as well as scientists and surgeons.
Christy Foreman with the FDA’s Center for Devices and Radiological Health, stated: “Well-designed registries in targeted product areas can enhance the public health and provide a cost-effective approach to clinical research for industry innovators. For HeartWare, registry data directly facilitated the development and availability of this new device.”
Source: Food and Drug Administration
Adderall Intake Instructions

Guide to Adderall intake instructions
The medication Adderall is prescribed by doctors to patients who suffer from attention deficit hyperactivity disorder (ADHD), as well as adults who are narcoleptic. Because of its potentially harmful and addictive side effects, Adderall's usage by patients should be closely monitored by physicians. To ensure that the drug does not become counter-productive, users should follow their doctors' guidelines at all times.
Adderall can be taken in the form of a once-a-day extended release tablet or multiple tablets to be taken two to three times a day. Regardless of the form, Adderall prescription strength can range anywhere from 2.5 milligrams (mg) to 60 milligrams. In any strength, the drug should only be taken in the form of an orally ingested tablet.
Adderall intake instructions for Children
Children who begin taking Adderall will start treatment with a 2.5 mg tablet, while adults will generally begin with a 5 mg dosage. Doctors will then monitor the drug's effects and increase the dosage as appropriate until an effective intake level is achieved. To prevent patients from abusing the medication, a prescription cannot be automatically refilled. Regularly scheduled appointments must be necessary to receive renewals to allow doctors to question patients concerning the presence of any side effects.
Adderall intake instructions: Addiction
The drug's amphetamine component makes it potentially addictive. Before prescribing Adderall, a doctor will inquire whether you or family members have a history of drug or alcohol abuse. If so, they are unlikely to prescribe the drug. However, even those without a history of substance abuse should be cautious and observe any indication they are developing a dependency.
Warning : Adderall intake instructions
There are no special conditions that must be observed when taking the drug. It is irrelevant whether the drug is consumed with or without food. For those taking tablets multiple times a day, it is recommended that doses are separated by four to six hours apiece. Patients who forget to take a dose are recommended to take it as soon as they remember, although it is not advisable to take two pills at once to catch up.
It is important not to abuse the drug; never take more of it or use it more frequently than as prescribed by your doctor. Some people may abuse Adderall by crushing and snorting it for recreational purposes. This is a dangerous act which can lead to many serious consequences, such as stroke or heart attack. Even when following a physician's instructions, it is important to be alert to any side effects which are observed.
Taking legal action: Adderall intake instructions
Patients who experience severe adverse effects may choose to pursue litigation to obtain damages for related medical expenses. Keep in mind that you will not be able to file a lawsuit if serious side effects occur due to abuse or overuse of the drug. If you have followed a doctor's directions and still experienced debilitating side effects, you will be potentially eligible to file a successful lawsuit. Consult a lawyer experienced in such cases rather than attempting to represent yourself in this complicated legal action.
Adderall Withdrawal

A Brief Overview of Adderall Withdrawal
The prescription drug Adderall carries many risks for those who take it to help alleviate ADHD and narcolepsy. While you should be aware of these side effects, those who follow a physician's usage instructions are not likely to experience Adderall withdrawal symptoms after discontinuing use.
Before prescribing the drug, a physician will ask for your medical history, including your history of alcohol or drug addiction in your family. If present, the doctor may be concerned of Adderall withdrawal and as a result, opt to prescribe an alternative amphetamine or medication. If usage is discontinued because of side effects or for other reasons, Adderall withdrawal is not likely to result in serious consequences.
Potential side effects will be observed even if you do not develop a chemical dependency to the drug. Adderall withdrawals are not as serious of a risk as severe side effects such as cardiac damage; however, prospective dependency should be monitored and dealt with promptly.
However, for patients who do not follow the guidelines for use, overuse can result in dependency. This means that Adderall withdrawals can lead to feelings of extreme physical discomfort. In addition, feelings of depression or suicidal urges may occur. This type of difficulty with Adderall withdrawal should be reported to a physician. While your use of the drug has been illegal, it is important to seek out medical assistance.
To help cope with such Adderall withdrawals, a physician may create a plan for steadily decreasing your dosage. Though the drug's effects on every person are different and often hard to predict, a physician will understand these consequences better. To help with any emotional difficulties stemming from Adderall withdrawal, a physician may choose to prescribe anti-depressants at this time. In extreme cases, Adderall withdrawals that occur following after a sudden discontinuation of use may necessitate resuming taking it at a lower dosage. You will then steadily decrease your use.
Studies concerning the use of the drug upon pregnant women are inconclusive. While Adderall withdrawals have been noted in rodent babies in clinical studies, no studies have been conducted to measure these effects on human babies. At the present, physicians are allowed to rely on their best judgment in deciding whether pregnant women should continue such treatment. If you are concerned about a baby developing a dependency and experiencing Adderall withdrawal after birth, consult with your physician.
Because the legal use of the drug is unlikely to result in this kind of condition, you should not be overly concerned. Similarly, Adderall withdrawals among abusers can be unpleasant but will not lead to death. Any discomfort experienced in the latter event is not grounds for litigation. Since your Adderall withdrawal was the direct consequence of willful abuse of the drug, your physician and the drug's parent company cannot be held responsible.
Yaz